Ángel Ramos, Ana Isabel Lorenzo, Sol Ferrán, Manuel Manrique
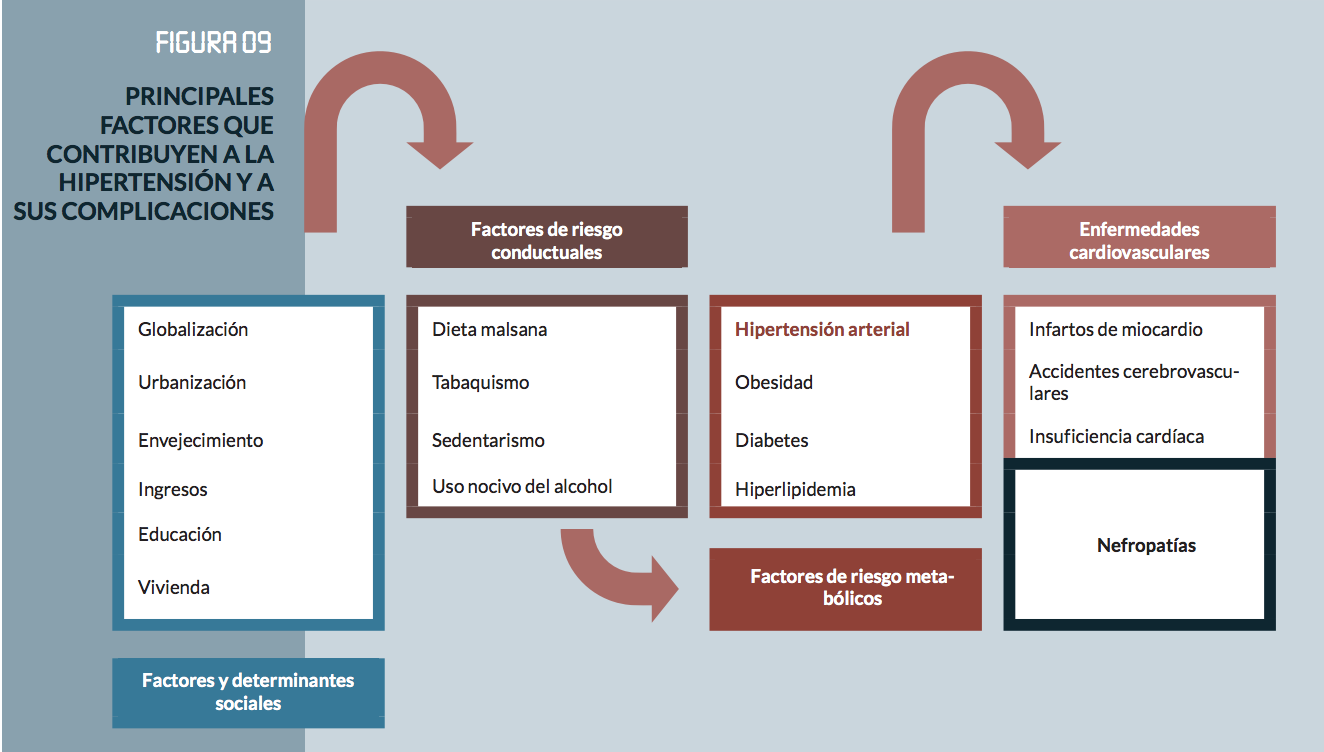
Metabolic risk factors
Diabetes
Ángel Ramos
Current evidence supports a powerful association between mild-subclinical hearing loss and diabetes mellitus (DM), both type I and type II. Besides, it seems glycemic control, its course and the existence of comorbidities or complications may be factors that predict developing a hearing loss. Nevertheless, it is noteworthy that the strongest statistical correlation between diabetes and hearing loss is found in young patients. This has been particularly studied in type I diabetes, to prevent confounding with age-related hearing loss. Besides, there seems to be a significant association between diabetes mellitus and increased risk of developing sudden hearing loss, particularly in patients with poor glycemic control.
Most of the papers reviewed evidence the implication of DM in hearing loss. Oh et al.1 found that age and DM were correlated with the prevalence of hearing loss. They showed that DM was a significant predictor of hearing loss (OR 1.398). This study proved that age-related hearing loss (presbycusis) starts in the high frequencies and gradually moves towards mid-range frequencies, and then the low frequencies.
In their study, Rolim et al.2 tried to prove whether patients with DM and hypertension suffered accelerated hearing loss, compared with individuals without these conditions. They performed two audiometric studies at 3–4-year intervals. They found statistically significant differences for the hypertensive group starting at 4kHz, and for the DM and hypertension group in the 500, 2kHz, 3kHz and 8kHz frequencies.
Besser et al.3 looked at studies that reveal higher prevalence of hearing loss in the high frequencies and in the mid-low frequencies in patients with altered basal glucose, compared with individuals with normal glucose levels, and individuals with high glycated hemoglobin.
The causal relation has not been proven yet. However, it has been suggested that the responsible physiopathology is mediated by cochlear damage, by circulatory disorders in the inner ear; a change to the endocochlear potential; retrocochlear hearing loss caused by auditory nerve neuritis, onset of diabetic neuropathy; and mitochondrial DNA mutations, alterations to the ATPase pump in the stria vascularis due to blood flow changes, which leads to an increased concentration of sodium in the endolymph.
References Diabetes
- Oh I-H, Lee JH, Park DC, Kim M, Chung JH, Kim SH, et al. Hearing Loss as a Function of Aging and Diabetes Mellitus: A Cross Sectional Study. PLoS One [Internet]. [cited 2020 Feb 5].
- Rolim LP, Samelli AG, Moreira RR, Matas CG, Santos I de S, Bensenor IM, et al. Effects of diabetes mellitus and systemic arterial hypertension on elderly patients’ hearing. Braz J Otorhinolaryngol. 2018 Nov 1;84(6):754–63.
- Besser J, Stropahl M, Urry E, Launer S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Vol. 369, Hearing Research. Elsevier B.V.; 2018. p. 3–14.
Hyperlipidemia
Ana Isabel Lorenzo
The effects of hyperlipidemia on hearing have been researched since 1964, when the first report observed a relation between hyperlipidemia, hearing loss and cardiovascular risk factors5. There have been many attempts to describe hearing loss associated with hyperlipidemia. It is induced by a pathological condition (hypertension, hyperlipidemia, or noise). It has been described as a variant of presbycusis3,4, sensorineural hearing loss in high frequencies3,5-7.
Some authors found no significant relation between hearing and the level of fasting triglycerides or cholesterol. They found that hearing loss was not greater in a population where fasting blood lipids were higher than in the control population7,8.
However, most authors have shown a relation between hyperlipidemia and blood flow. The spiral lamina and the stria vascularis are the better vascularized portions of the inner ear, while using the same amount of oxygen the retina does9. The sensorineural hearing loss following hyperlipidemia can be associated with atherosclerosis, microvascular embolism, hereditary factors, hypertension and aging7. The cochlear vascular walls regulate the cochlear blood flow and produce nitric oxide, a vasodilator10. LDL cholesterol can stop cochlear blood flow by blocking the production of nitric oxide in the vascular wall and reducing the motility of outer hair cells1,3,11,21-24. It has been proposed that hypertriglyceridemia, hypercholesterolemia, and hyperfibrinogenemia can lead to decreased cochlear blood flow because of hyperviscosity and vascular defects due to atherosclerosis3,21.
Experimental studies with lipid-rich diets revealed pathological changes to the stria vascularis and the outer hair cells, with additive effects when associated with hypertension and high cholesterol1. Satar et al2 conducted an experimental study in pigs to compare a control group fed with the regular diet, and a cholesterol group, which was fed 1 gr. of cholesterol per day for 4 months. The control group presented normal cochlear structures with regular hearing thresholds, whereas the cholesterol group had profound edema in the marginal layer of the stria, and mild edema in the outer hair cells, in line with the data from the brainstem auditory response, which revealed changes to hearing sensitivity.
Gatehouse et al.12 researched the connection between hyperviscosity and its effects on the auditory function. In light of that study, it was proven that hyperviscosity can lead to sensorineural hearing loss in higher frequencies, such as 2-4 KHz. It concluded that hearing dysfunction due to hyperlipidemia may be attributed to hyperviscosity, vascular occlusion and greater sensitivity to noise.
Sikora et al.32 argued that a diet high in cholesterol could increase the sensitivity to noise and provoke hearing loss in rodents. Axelsson and Lindgren14 observed that people who worked in noisy environments and had elevated serum cholesterol had slightly higher risk of developing sensorineural hearing loss in high frequencies. Erdem et al3 described that the width of OAE shrank to 4 KHz in patients with hypertriglyceridemia, which is compatible with the pattern of hearing loss seen in hyperviscosity and greater sensitivity to noise in patients with hyperlipidemia.
Suzuki et al.15 observed that patients with elevated high-density lipoprotein (HDL) cholesterol had better hearing levels in 2 and 4 KHz.
In January 2020, Wang et al16 concluded that elevated non-HDL cholesterol levels in blood are associated with higher risk of sudden sensorineural hearing loss, and that non-HDL cholesterol levels can be a biomarker that predicts the risk of sudden hearing loss. In the same way, Ballesteros17 and Aimoni18 propose that hypercholesterolemia and BMI19 are associated with sudden sensorineural hearing loss.
In 2009, Cai et al20 conducted an experimental study in mice and verified that the drugs used to prevent and treat atherosclerosis, such as simvastatin, are otoprotective in mice. In the same way, based on the outcome of an experimental study with 34 mice, Syka and col.25,22 suggested that atorvastatin could moderate the course of age-related hearing loss in mice.
Several studies have evaluated the effect of statins on hearing, based on the argument that statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA), associated with lower plasmatic viscosity and epithelium-derived nitric oxide-mediated vasodilation22-24. Leaving aside the reducing effect of cholesterol, statins affect the vascular endothelium and increase the NO synthesis, decrease blood viscosity, and regulate cochlear microcirculation24,22. These observations suggest that statins can reduce the microvascular effects of hyperlipidemia19,22. Yucel et al22 published a study about the effect of statins on hearing, and the subjective sensation of tinnitus. They observed that the groups of statins improved in the 6000Hz frequency, but there were no statistically significant differences among them in the audiometry. However, a 2 dB improvement, statistically significant, was found in hearing thresholds after using statins at 6000 Hz. It is too small a difference to be clinically significant. Besides, this outcome could be attributed to the audiometric technician or the patient.
In contrast with these statements, some reports describe that statins may harm hearing. Chung SD et al.27 examined the association between sudden sensorineural hearing loss (SSNH) and the use of statins in a study. They detected a statistically significant relation between SSNH and the prior use of statins, irrespective of the regular use or not of statins. Olzowy and col.26 conducted a double blind, clinical trial in which they studied the effect of atorvastatin on hearing loss and tinnitus in older patients. They did not find statistically significant differences in hearing thresholds. However, they found that they could diminish the subjective sensation of tinnitus.
On a different note, metabolic syndrome (MS) is associated with an increase in hearing loss28-30. MS is defined as the existence of 3 or more of the following components:
- Waist circumference ≥ 102 cm in men, and ≥ 88 cm in women.
- Hypertriglyceridemia ≥ 150mg/dl.
- Low levels of HDL < 40mg/dl in men and < 50mg/dl in women.
- High blood pressure: systolic pressure ≥ 130 mmHg or diastolic pressure ≥ 85 mmHg.
- Elevated glycemia ≥ 20mg/dl28.
In 2015, Sun et al28 found that the presence of the metabolic syndrome was significantly associated with hearing loss, both in high and low frequencies. They also observed a positive relation between hearing loss and a greater number of MS components, particularly low HDL level, and high triglyceride level, which revealed a strong association with the increase in hearing loss. In much the same way, Shin et al29 concluded that the greater the number of MS components, the greater the risk of hearing loss. In 2019, Zhang et al31 argued that MS has negative impact on the recovery from sudden sensorineural hearing loss, and that the prognosis of these patients worsens the more MS components they have.
Because of all this, it is concluded that:
- There is a direct relation between dyslipidemia and sensorineural hearing loss in high frequencies.
- Dyslipidemia increases hyperviscosity, diminishes vasodilation as nitric oxide decreases, and diminishes cochlear blood flow.
- Dyslipidemia increases the risk of sensorineural hearing loss for individuals exposed to noise.
- Elevated HDL seems to protect from hearing loss.
- Statins are protective and can minimize the microvascular effects of dyslipidemia.
References Hyperlipidemia
- Chávez-Delgado ME, Vázquez-Granados I, Rosales-Cortés M, Velasco-Rodríguez V. Disfunción cócleo-vestibular en pacientes con diabetes mellitus, hipertensión arterial sistémica y dislipidemia. Acta Otorrinolaringol Esp. 2012;63(2):93–101.
- Satar B, Ozkaptan Y, Sürücü HS, Oztürk H. Ultrastructural effects of hypercholesterolemia on the cochlea. Otol Neurotol. 2001;22(6):786–789.
- Erdem, T., Ozturan, O., Miman, M. et al. Exploration of the early auditory effects of hyperlipoproteinemia and diabetes mellitus using otoacoustic emissions. Eur Arch Otorhinolaryngol 260, 62–66 (2003)
- Cunningham DR, Goetzinger CP (1974) Extra-high frequency hearing loss and hyperlipidemia. Audiology 13: 470–484
- Rosen S, Plester D, El-Mofty A, Rosen HV (1964) Relation of hearing loss to cardiovascular disease. Trans Am Acad Ophtal- mol Otolaryngol 68: 433–444
- Spencer JT (1975) Hyperlipoprotenemia and inner ear disease. Otolaryngol Clin North Am 8: 483–492
- Jones NS, Davis A (1999) A prospective case-controlled study of patients presenting with idipatic sensorineural hearing loss to examine the relationship between hyperlipidemia and sensorineural hearing loss. Clin Otolaryngol 24: 531–536
- Jorgensen M, Buch H (1961) Studies on inner-ear function and cranial nerves in diabetes. Acta Otolaryngol 74: 373–381
- Spencer JT (1973) Hyperlipoprotenemia in the etiology of in- ner ear disease. Laryngoscope 83: 639–678
- Brechtelsbauer P, Nuttal A, Miller J (1994) Basal nitric oxide production in regulation of cochlear blood flow. Hear Res 77: 38–42
- Seiler C, Hess OM, Buechi M, Suter TM, Krayenbuelh HP (1993) Influence of serum cholesterol and other coronary risk factors on vasomotion of angiographically normal coronary ar- teries. Circulation 88: 2139–2148
- Gatehouse S, Gallcher JEJ, Lowe GDO, Yarnel JWG, Hutton RD, Isy?ng I (1989) Blood viscosity and hearing levels in the caerphilly collaborative heart disease study. Arch Otolaryngol Head Neck Surg 115: 1227–1230
- Seiler C, Hess OM, Buechi M, Suter TM, Krayenbuelh HP (1993) Influence of serum cholesterol and other coronary risk factors on vasomotion of angiographically normal coronary ar- teries. Circulation 88: 2139–2148
- Axelsson A, Lindgren F (1985) Is there a relationship betwen hypercholesterolemia and noise-indused hearing loss? Acta Otolaryngol (Stockh) 100: 379–386
- Suzuki K, Kaneko M, Murai K (2000) Influence of serum lipids on auditory function. Laryngoscope 110: 1736–1738
- Wang S, Ye Q, Pan Y. Serum non-high-density lipoprotein cholesterol is associated with the risk of sudden sensorineural hearing loss. Medicine (Baltimore). 2020;99(7):e19175.
- Ballesteros F, Alobid I, Tassies D, et al. Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors? Audiol Neurootol 2009;14:139–45.
- Aimoni C, Bianchini C, Borin M, et al. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol 2010;15:111–5.
- Lee JS, Kim DH, Lee HJ, et al. Lipid profiles and obesity as potential risk factors of sudden sensorineural hearing loss. PLoS One. 2015;10(4):e0122496. Published 2015 Apr 10. doi:10.1371/journal.pone.0122496
- Cai Q, Du X, Zhou B, et al. Effects of simvastatin on plasma lipoproteins and hearing loss in apolipoprotein E gene-deficient mice. ORL J Otorhinolaryngol Relat Spec. 2009;71(5):244–250. doi:10.1159/000236014
- Malgrange B, Varela-Nieto I, de Medina P, Paillasse MR. Targeting cholesterol homeostasis to fight hearing loss: a new perspective. Front Aging Neurosci. 2015;7:3. Published 2015 Jan 29.
- Yücel H, Yücel A, Arba? H, Cure E, Eryilmaz MA, Özer AB. Effect of statins on hearing function and subjective tinnitus in hyperlipidemic patients. Rom J Intern Med. 2019;57(2):133–140.
- Maron DJ., Fazio S., Linton MF. Current perspectives on Statins. Circulation. 2000; 18;101(2):207-13.
- Feron O., Dessy C., Desager JP., Balligand JL. Hydroxy-methylglutaryl- coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103(1):113-8.
- Syka J., Ouda L., Nachtigal P., Solichova D., Semecky? V. Atorvastatin slows down deterioration of inner ear function with age in mice. Neurosci Lett. 2007;411(2):112-6.
- Olzowy B., Canis M., Hempel JM., Mazurek B., Suckfull M. Effect of atorvastatin on progression of sensorineural hearing loss and tinnitus in the elderly. Otol Neurotol. 2007;28(4):455-8.
- Chung SD., Chen CH., Hung SH., Lin HC., Wanglh. A population-based study on the association between statin use and sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2015;152(2):319-25.
- Sun YS, Fang WH, Kao TW, et al. Components of Metabolic Syndrome as Risk Factors for Hearing Threshold Shifts. PLoS One. 2015;10(8):e0134388. Published 2015 Aug 6.
- Shim HS, Shin HJ, Kim MG, et al. Metabolic syndrome is associated with hearing disturbance. Acta Otolaryngol. 2019;139(1):42–47.
- Han X, Wang Z, Wang J, et al. Metabolic syndrome is associated with hearing loss among a middle-aged and older Chinese population: a cross-sectional study [published correction appears in Ann Med. 2018 Nov;50(7):636]. Ann Med. 2018;50(7):587–595.
- Zhang Y, Jiang Q, Wu X, Xie S, Feng Y, Sun H. The Influence of Metabolic Syndrome on the Prognosis of Idiopathic Sudden Sensorineural Hearing Loss. Otol Neurotol. 2019;40(8):994–997.
High Blood Pressure
Ana Isabel Lorenzo, Sol Ferrán, Manuel Manrique
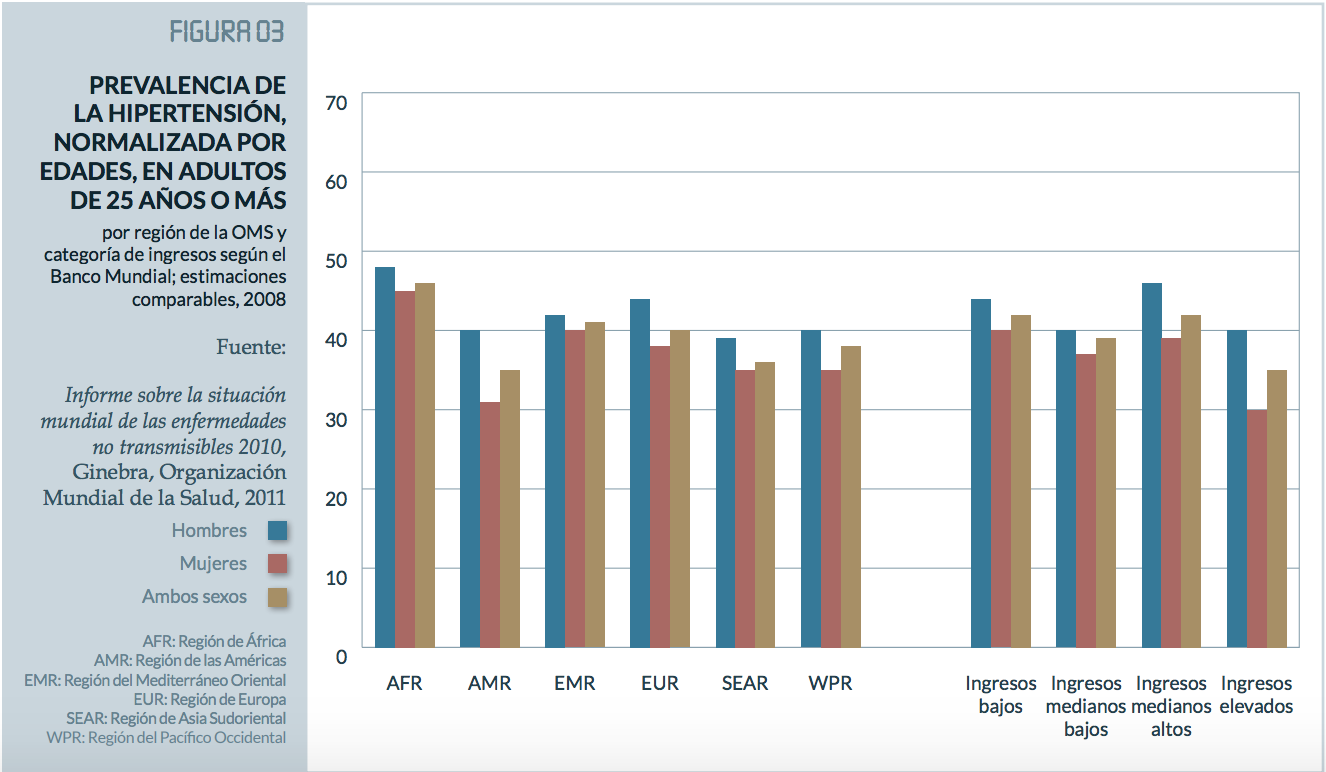
High blood pressure is the most frequent chronic disease in adults. The global prevalence of hypertension, or high blood pressure (defined as systolic and/or diastolic blood pressure equal or greater than 140/90 mm Hg) in adults over age 18 was 22% in 2014, according to the latest WHO report1. Hypertension is most prevalent in Africa, where 46% of adults over age 25 suffer it. The lowest prevalence is in the American Hemisphere, at 35%1. Hypertension tends to be less prevalent in high-income countries (35%), compared with 40% in lower income countries1. There are 11 million people (36.7% according to the WHO) who suffer this disease in our country.
According to the WHO, over 5% of the world’s population, that is 466 million people, suffer from a disabling hearing loss (432 million adults and 34 million children). It is estimated that more than 900 million people, or one in ten people, will suffer a disabling hearing loss by 2050. A disabling hearing loss is defined as a hearing loss of more than 40 decibels (dB) in the better-hearing ear in adults and a hearing loss of more than 30 dB in the better-hearing ear in children. Most people with a disabling hearing loss live in mid and low-income countries. Approximately a third of the people over age 65 are hearing impaired. Prevalence in this age group is higher in Southern Asia, Asia Pacific, and Sub-Saharan Africa. Deafness affects approximately 3 million people over the age of 65 in Spain and between one and five newborn children per thousand are born with some degree of hearing loss.
Rosen and Olin (1964) found in a study that people between ages 40-59 with a heart disease had poorer hearing sensitivity than people within that age group and no heart disease5,35
The hypothesis of the harmful effect of high blood pressure on the cochlea and the vestibular system was developed in 1968 in a study conducted by Hansen et al2. Multiple studies have been conducted since, with disparate outcomes3,35.
Experimental studies with animals have concluded that maintaining the proper cochlear blood flow is critical for the cochlear function and any reduction to the blood supply would limit cochlear function and could result in irreversible damage to all the cochlea5.
Tan et al6 set forth in their study about hearing loss and hypertensive retinopathy that patients with hypertensive retinopathy had poorer hearing thresholds in high frequencies. They concluded that vascular compromise is observed in patients with poorly controlled hypertension and associated with hearing loss in the high frequencies. The authors suggest that the hypertensive microangiopathy of the cochlea and retina produce hearing loss and loss of visual acuity, respectively.
Nazar et al,7 undertook a study where they controlled and treated 217 patients with hypertension and no diabetes mellitus, and no previous exposure to noise or ototoxic drugs for 3 years. They were compared with a healthy control group. The comparative analysis of the results of both groups did not yield significant differences. One may say that the population with well controlled chronic hypertension and the general population have similar audiometric profiles.
On a different note, Marchiori et al9 studied the association between high blood pressure and hearing loss. They concluded there is a significant association between high blood pressure and hearing loss. They suggest hypertension is a factor that accelerates the deterioration of the hearing system with age9,10,11. They also described that hypertension, older age, male gender, and exposure to noise are independent risk factors for hearing loss6,34. Chen et al4 studied the relation between high blood pressure and auditory conditions in older people. They state that hypertension associated with high levels of triglycerides and cholesterol worsen the hearing of older people. Auditory conditions in older people may be the result of long-term hypertension and its complications.
Borg et al8 conducted an experimental study in which two groups of rats (normotensive and hypertensive, respectively) were exposed to noisy environments and concluded that the group of normotensive rats barely lost any hair cells, compared with the hypertensive rats, which lost more hair cells. Tachibana et al12 published another study involving rats that revealed the primary site of cochlear impairment: the stria vascularis, followed by the organ of Corti.
Makishima et al13 studied the histopathology of 80 temporal bones from 40 patients over age 50, paying particular attention to angiosclerotic changes. The histopathological findings correlated with the audiometric and manometric records obtained when the patients were alive. They proved there is a remarkable correlation between the narrowing of the internal auditory artery, the spiral ganglion atrophy and hearing loss.
Then, Esparza et al.14 in their study on inner ear dysfunction and blood pressure suggested that hypertensive patients may suffer from a cochlear dysfunction associated with the vascular disease resulting from their high blood pressure. High blood pressure may be silent and reveal no clear evidence of vestibular dysfunction.
Several studies show how hearing loss occurs with age and is associated with inappropriate microcirculation due to vascular occlusion, embolus, hemorrhage or vasospasms15. Other authors ascribe the hearing loss to the hyperviscosity or microangiopathy associated with DM, hypertension, and the association of several pathologies.11,15,32
There are multiple factors to the physiopathology of hearing loss associated with high blood pressure. Damage to a highly vascularized stria vascularis has been proposed as an indirect hearing loss mechanism. The normal function of the stria vascularis is needed to maintain homeostasis in the inner ear. The loss of stria tissue stops the production and retake of potassium, which translate in impaired signal transduction and probably increases the production of free radicals in the inner ear17,18. All this produces oxidative stress and hypoxia, and the subsequent capillary damage.
Additionally, the stria vascularis corresponding to high cochlear frequencies is particularly vulnerable to capillary deterioration and thinning of the basal membrane, due to alterations to the cochlear blood flow. This set of homeostatic changes resulting from high blood pressure may amplify the negative effect of extrinsic factors, such as excessive exposure to noise, and lead to an accelerated hearing loss in high frequencies in an area of the cochlea that is tonotopically organized and is also subject to age-related hearing loss17.
Reed et al17 observed a strong association between high blood pressure in middle-age patients and high-frequency hearing loss. Using histochemical methods, Carrasco et al15 proved the existence of adrenergic nervous terminations in the terminal arterioles and radials which branches feed the stria vascularis of mice’s cochleae. This study proved the presence and distribution of adrenergic receptors in cochlear microcirculation and observed arteriolar vasoconstriction following the intravascular administration of alpha-adrenergic agonists as well.
A study by Duck et al16 supports the hypothesis that cochlear damage is more intense with concomitant hypertension32. Similarly, this hypothesis was verified in an animal study, given the significant rise in the average loss of hair cells in a group of hypertensive rats with insulin-dependent DM, compared with normotensive rats with insulin-dependent DM. In the same way, there were statistically significant differences in the hearing loss of patients with insulin-dependent DM and hypertension, and normotensive patients with insulin-dependent DM.
Based on these results, it might seem reasonable to support the hypothesis that hypertension is a risk factor for high-frequency hearing loss. Besser et al37 studied the relationship with cardiovascular diseases and found that the odds of hypertensive individuals were 1.5 greater than those of healthy individuals. They even mention a lineal relation between blood pressure and hearing loss, where hearing loss increases by 0.03 dB for every 1mmHg.
In the same way, there are several studies covering the association between exposure to noise and developing hypertension and hearing loss32. In 1990, Talbott et al,19 described the existing relation between exposure to occupational noise, noise-induced hearing loss and hypertension.
In 2011, Chan et at20 undertook a cross-sectional study. They set forth that there is a link between a prolonged exposure to occupational noise and a higher incidence of hypertension. Chan explains that noise is a psychological stress factor. As such, it activates the hypothalamus-hypophysis-adrenal axis and the sympathetic nervous system. This rises the levels of adrenalin, noradrenalin, and cortisol, the three hormones that influence the regulation of blood pressure.24,25,26
Many studies have suggested that exposure to occupational noise is linked to consistently high blood pressure or significantly greater risk of hypertension11,21-23,28-30,32,34. Others, however, do not find this relation to be statistically significant19,27. This difference may be due to the degree of hearing protection of workers: noise levels outdoors are not the actual intensity of noise exposure for the inner ear.20
Tarter et al31 found that exposure to noise levels ≥ 85 dB for more than 5 years is linked to a 28.3 dB HL at 4 kHz among workers in a car assembly.
In the same way, Chan describes in his study that the prevalence of hypertension is greater among groups with hearing loss in mid to high frequencies. There is higher prevalence of hypertension in populations over age 40. He concludes that a noise-induced hearing loss is significantly linked to hypertension after controlling for potential concomitant risks. Hearing loss in high frequencies is a good biomarker of occupational noise exposure. Hearing thresholds above 15dB in 4kHz or 6kHz over more than 5 years are linked to increased risk of hypertension, but this risk disappears when the ears are protected (plugs).
Because of all this, it is concluded that:
- Hypertension is an important risk factor for sensorineural hearing loss in high frequencies.
- Presbycusis is associated with inappropriate microcirculation. Hypertension causes a microangiopathy that compromises cochlear blood flow and intensifies damage to the cochlea by boosting age-related hearing loss.
- Consistent exposure to occupational noise is linked to consistently high blood pressure or greater risk of hypertension. This risk lowers with hearing protection.
- The general population and well-controlled chronic hypertensive individuals have similar audiometric profiles.
- Hypertension associated with other comorbidities, such as dyslipidemia or diabetes mellitus impairs the hearing of older people even more.
References High Blood Pressure
- Organización Mundial de la Salud. Informe sobre la situación mundial de las enfermedades no transmisibles 2014.
- Hansen CC. Perceptive hearing loss and arterial hypertension. Arch Otolaryngol. 1968;87(2):119–122.
- Przewo?ny T, Gójska-Grymaj?o A, Kwarciany M, G?secki D, Narkiewicz K. Hypertension and cochlear hearing loss. Blood Press. 2015;24(4):199–205.
- Chen YL, Ding YP. Relationship between hypertension and hearing disorders in the elderly. East Afr Med J. 1999;76(6):344–347.
- Torre P 3rd, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. The association between cardiovascular disease and cochlear function in older adults. J Speech Lang Hear Res. 2005;48(2):473–481.
- Tan TY, Rahmat O, Prepageran N, Fauzi A, Noran NH, Raman R. Hypertensive retinopathy and sensorineural hearing loss. Indian J Otolaryngol Head Neck Surg. 2009;61(4):275–279.
- Nazar J, Otalora F, Acevedo l. Audición del paciente hipertenso crónico controlado Rev. otorrinolaringol. cir. cabeza cuello;52(2):97-104, ago. 1992.
- Borg E. Noise-induced hearing loss in normotensive and spontaneously hypertensive rats. Hear Res. 1982;8(2):117–130.
- de Moraes Marchiori LL, de Almeida Rego Filho E, Matsuo T. Hypertension as a factor associated with hearing loss. Braz J Otorhinolaryngol. 2006;72(4):533–540.
- Agarwal S, Mishra A, Jagade M, Kasbekar V, Nagle SK. Effects of hypertension on hearing. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 3):614–618.
- Wang B, Han L, Dai S, et al. Hearing Loss Characteristics of Workers with Hypertension Exposed to Occupational Noise: A Cross-Sectional Study of 270,033 Participants. Biomed Res Int. 2018;2018:8541638.
- Tachibana M, Yamamichi I, Nakae S, Hirasugi Y, Machino M, Mizukoshi O. The site of involvement of hypertension within the cochlea. A comparative study of normotensive and spontaneously hypertensive rats. Acta Otolaryngol. 1984;97(3-4):257–265.
- Makishima K. Arteriolar sclerosis as a cause of presbycusis. Otolaryngology. 1978;86(2):ORL322–ORL326.
- Esparza CM, Jáuregui-Renaud K, Morelos CM, et al. Systemic high blood pressure and inner ear dysfunction: a preliminary study. Clin Otolaryngol. 2007;32(3):173–178.
- Carrasco VN, Prazma J, Faber JE, Triana RJ, Pillsbury HC. Cochlear microcirculation. Effect of adrenergic agonists on arteriole diameter. Arch Otolaryngol Head Neck Surg. 1990;116(4):411–417.
- Duck SW, Prazma J, Bennett PS, Pillsbury HC. Interaction between hypertension and diabetes mellitus in the pathogenesis of sensorineural hearing loss. Laryngoscope. 1997;107(12 Pt 1):1596–1605.
- Reed NS, Huddle MG, Betz J, et al. Association of Midlife Hypertension with Late-Life Hearing Loss. Otolaryngol Head Neck Surg. 2019;161(6):996–1003.
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83.
- Talbott EO, Findlay RC, Kuller LH, et al. Noise-induced hearing loss: a possible marker for high blood pressure in older noise-exposed populations. J Occup Med. 1990;32(8):690–697.
- Chang TY, Liu CS, Huang KH, Chen RY, Lai JS, Bao BY. High-frequency hearing loss, occupational noise exposure and hypertension: a cross-sectional study in male workers. Environ Health. 2011;10:35. Published 2011 Apr 25.
- Rosenlund M, Berglind N, Pershagen G, Järup L, Bluhm G. Increased prevalence of hypertension in a population exposed to aircraft noise. Occup Environ Med. 2001;58(12):769–773.
- Leon Bluhm G, Berglind N, Nordling E, Rosenlund M. Road traffic noise and hypertension. Occup Environ Med. 2007;64(2):122–126.
- Jarup L, Babisch W, Houthuijs D, et al. Hypertension and exposure to noise near airports: the HYENA study [published correction appears in Environ Health Perspect. 2008 Jun;116(6):A241]. Environ Health Perspect.
- Spreng M. Central nervous system activation by noise. Noise Health. 2000;2(7):49–58.
- Babisch W. The Noise/Stress Concept, Risk Assessment and Research Needs. Noise Health. 2002;4(16):1–11.
- Ising H, Kruppa B. Health effects caused by noise: evidence in the literature from the past 25 years. Noise Health. 2004;6(22):5–13.
- Hirai A, Takata M, Mikawa M, et al. Prolonged exposure to industrial noise causes hearing loss but not high blood pressure: a study of 2124 factory laborers in Japan. J Hypertens. 1991;9(11):1069–1073.
- Kuang D, Yu YY, Tu C. Bilateral high-frequency hearing loss is associated with elevated blood pressure and increased hypertension risk in occupational noise exposed workers. PLoS One. 2019;14(9):e0222135. Published 2019 Sep 5.
- Zhou F, Shrestha A, Mai S, et al. Relationship between occupational noise exposure and hypertension: A cross-sectional study in steel factories. Am J Ind Med. 2019;62(11):961
- Liu J, Xu M, Ding L, et al. Prevalence of hypertension and noise-induced hearing loss in Chinese coal miners. J Thorac Dis. 2016;8(3):422–429.
- Tarter SK, Robins TG. Chronic noise exposure, high-frequency hearing loss, and hypertension among automotive assembly workers. J Occup Med. 1990;32:685–689.
- Besser J, Stropahl M, Urry E, Launer S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hear Res. 2018;369:3–14.
- Umesawa M, Sairenchi T, Haruyama Y, Nagao M, Kobashi G. Association between hypertension and hearing impairment in health check-ups among Japanese workers: a cross-sectional study. BMJ Open. 2019;9(4):e028392. Published 2019 Apr 24.
- Meneses-Barriviera CL, Bazoni JA, Doi MY, Marchiori LLM. Probable Association of Hearing Loss, Hypertension and Diabetes Mellitus in the Elderly. Int Arch Otorhinolaryngol. 2018;22(4):337–341.
- Bao M, Song Y, Cai J, Wu S, Yang X. Blood Pressure Variability Is Associated with Hearing and Hearing Loss: A Population-Based Study in Males. Int J Hypertens. 2019;2019:9891025. Published 2019 Feb 3.
- Rosen S, Plester D, El-Motfy A, Rosen HV. Relation of hearing loss to cardiovascular disease. Trans Am Acad Ophthalmol Otolaryngol. 1964; 68:433-444.
- Besser J, Stropahl M, Urry E, Launer S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Vol. 369, Hearing Research. Elsevier B.V.; 2018. p. 3–14.
Metabolic Risk Factors: Conclusions
In conclusion, cardiovascular factors related to hearing loss, particularly age-related hearing loss, have been studied in depth. Many significant associations have been found, particularly with diabetes mellitus and high blood pressure. However, we also find studies that do not support this association, and in some cases, they are not clinically relevant, despite being statistically significant.

